13 July 2023
The highlights below are from the quarterly meeting of the Vaccine Advisory Committee (VAC) on July 13, 2023.
COVID-19
- Fall/Winter 2023 COVID-19 Vaccine: The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted unanimously to recommend a 2023-2024 Formula update of the current COVID-19 vaccine composition to a monovalent XBB-lineage; FDA advised manufacturers to develop vaccines with a monovalent XBB.1.5 composition.
- Covid-19 Vaccine Ordering Shut-Off: In anticipation of commercialization this fall, Washington's Immunization Information System (IIS) COVID-19 vaccine ordering will be terminated at 4 pm PST on Wednesday, August 2, 2023. The U.S. Government COVID-19 Vaccine Distribution Program will provide direction to those participating in the COVID Vaccine Program as the USG stops distributing COVID vaccines through the current ordering system.
- Covid-19 Access Considerations: The Washington Department of Health (DOH) has received concerns from public that some patients have been turned away from receiving COVID-19 vaccine doses from providers because they are not established patients at those facilities or because of their insurance plan/coverage. Important reminder: Providers enrolled in the CDC-DOH COVID-19 vaccination program remain subject to the terms of the CDC COVID-19 Vaccination Program Provider Agreement, until the COVID-19 vaccine is commercialized this fall, at which time the COVID-19 Vaccine Provider Agreements will be nullified. Any questions should be sent to COVID.vaccine@doh.wa.gov
- HHS Bridge Access Program: The "Bridge Access Program For COVID-19 Vaccines and Treatments" is a program currently in planning stages by the Department of Health and Human Services (HHS). Its purpose is to ensure broad access to COVID-19 vaccines and treatments for under- and uninsured adults once these products are commercialized. All providers enrolled in the Adult Vaccine Program (AVP) will be eligible to participate in vaccine ordering and distribution through the Bridge Program. The CDC will procure and distribute COVID-19 vaccines under the Bridge Program to awardees. (They are still figuring out details surrounding vaccine ordering and allocation.) The CDC will carry out a needs assessment with awardees in July 2023. The results of this assessment will allow CDC to determine the quantities and types of vaccines that should be purchased for the program. Depots will not be permitted under the Bridge Access Program. More details to come in future! Contact PolicyISDBridge@cdc.gov with additional questions related to the Bridge Program.
Care-A-Van (CAV)
The Care-a-Van (CAV) is a mobile health clinic that serves people across Washington state. CAV works closely with community partners and local health jurisdictions (LHJ) or departments to increase access to vaccine for priority communities. The CAV currently offers the following vaccines: COVID-19, Mpox, Childhood Vaccines, Flu

Power of Providers (POP)
Washington's DOH POP is holding a Virtual Recognition Event on Wednesday, July 19, from 12:30 to 1:30 pm PT. This event will feature words of appreciation from Governor Jay Inslee and Secretary of Health Dr. Umair Shah; highlights of POP member efforts over the past 2 years (including from our very own Andrew Simon, ND!); and words of inspiration from Dr. Kira Mauseth on recognizing our collective achievements.
Meeting Updates from Advisory Committee on Immunization Practices (ACIP)
- RSV in adults
- FDA licensed 2 RSV vaccines in May (Arexvy and Abrysvo)
- Manufacturers provided VE
- Cost effectiveness reviewed
- Considerations for favoring vaccination include comorbid conditions and advanced age, in addition to other variables
- ACIP recommendation: Adults 60 years of age and older may receive a single dose of RSV Vaccine, using shared clinical decision making
- Polio
- Summarized work group deliberations on adult polio
- Clarified and updated inactivated polio (IPV) recommendations for adults
- ACIP Recommendation for adults incompletely immunized against polio: Adults (age18 or older) who are known or suspected to be unvaccinated or incompletely vaccinated against polio should complete a primary series with IPV.
- ACIP recommendation for the option of one adult polio booster, if at risk of exposure: Adults who received a primary series of trivalent OPV (tOPV) or IPV in any combination and who are at increased risk of poliovirus exposure may receive another dose of IPV.
- Available data do not indicate the need for more than a single lifetime booster dose with IPV for adults.
- SOURCE: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-POLIO-Kidd-Jun-2023.pdf
- Influenza
- All Vaccines will be quadrivalent for the 2023-2024 influenza Season
- 2023-24 composition includes updated influenza A(H1N1) pdm09 components
- Addressed egg-allergy with following guidance:
- All persons aged 6 months and older with egg allergy should receive influenza vaccine
- Any influenza vaccine (egg based or non-egg based) that is otherwise appropriate for the recipient’s age and health status can be used
- PCV
- Either PCV15 or PCV20 is recommended for all children age 2 through 23 months according to currently recommended PCV dosing and schedules
- For older children with an incomplete PCV vaccination status, use of either PCV15 or PCV20 according to currently recommended PCV dosing and schedule is recommended for:
- Healthy children age 24 through 59 months
- Children with specified risk conditions age 24 through 71 months
- For children age 2 through 18 years with any risk condition who have received all recommended doses before age 6 years:
- If they received at least 1 dose of PCV20: No additional doses of any pneumococcal vaccine are indicated. This recommendation may be updated as additional data become available.
- If they received PCV13 or PCV15 (but no PCV20): ACIP recommends a dose of PCV20 alone, or PPSV23 using previously recommended doses and schedule
- For children ages 6 through 18 years with any risk condition who have not received any dose of PCV13, PCV15, or PCV20, give a single dose of PCV20 alone, or PCV15 followed by a dose of PPSV23 at least 8 weeks later, if not previously given
- SOURCE: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/04-Pneumococcal-Kobayashi508.pdf
- There is an app available called PneumoRecs Vax Advisor
- The PneumoRecs VaxAdvisor mobile app helps vaccination providers quickly and easily determine which pneumococcal vaccines a patient needs and when.
- The app incorporates recommendations for all ages so internists, family physicians, pediatricians, and pharmacists alike will find the tool beneficial.
- COVID-19
- Current COVID-19 Vaccine epidemiology and vaccine effectiveness reviewed
- Uptake of bivalent continues to be low
- Plans are underway for the transition to routine commercial use of COVID-19 vaccines
- A new monovalent COVID-19 vaccine containing a current variant XBB.1.5 has been recommended by FDA for fall 2023
- Once updated vaccines are licensed or authorized by FDA, ACIP will review evidence to inform its recommendations
- SOURCE: https://www.cdc.gov/vaccines/acip/index.html
Studies in Vaccine Safety Data (VSD)
The VSD provides an opportunity to study possible trends associated with vaccines. Studies highlighted in ACIP meeting:
- Antigens and non-targeted infections: addressed public concerns over vaccines overloading immune system and found no association
- Schedules and type 1 diabetes (T1DM): addressed public concerns over possibility of vaccines causing autoimmune disorders and found vaccine schedule not associated with increased risk of T1DM
- Aluminum and asthma: addressed concerns over possibility of vaccines causing increased risk of allergic disorders including asthma and some associations although large limitations noted such as lack of data on dietary/environmental exposures. Large Danish study found no support for an association between aluminum in vaccines and asthma by 5 years of age
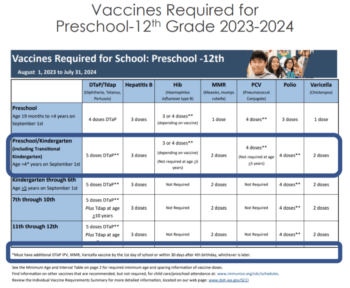
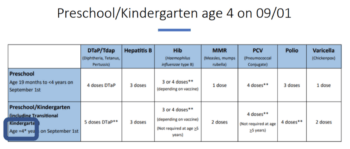
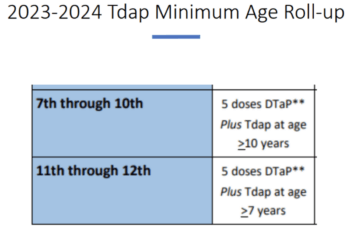
School and Childcare Immunization Requirements

~Mary Koehnke ND, FABNP | WANP Liaison to WA DOH VAC
